June 2001Creating a Pure Vacuum from Water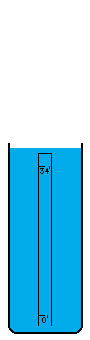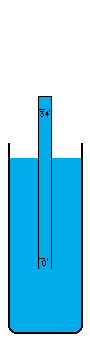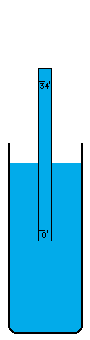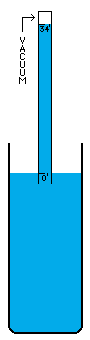
I'm not sure how vacuums are presently created. A number of devices depend on vacuums, so there's no question that someone somewhere has derived a pretty good way to do it. I think the most common way is with simple fans. Fans simply blow the air out of a contained volume and the air density decreases. However, this method cannot possibly create a perfect vacuum. The decrease in density must drop off asymptotically without every reaching zero. I believe there is an easy way to create an absolutely perfect vacuum however (maybe I'm wrong, maybe it doesn't work, I don't know). This is a simply physics experiment that I learned about a long time ago. If you submerge a glass in a bowl of water, turn it upside down, and pull it up out of the water, it will come up completely filled with water even though it is above the waterline. The reason for this is simple. There was no air in the glass to begin with and no way for air to get into the glass, so the external air pressure pushing down on the water in the bowl will push water up into the glass. Now, when the force of the external air equals the force of the water's mass trying to descend out of the glass, the two forces are balanced. This depth is thirty-three feet in sea water and thirty-four feet in fresh water. Beyond that point, the air pressure isn't powerful enough to push the column of water any higher. I believe that pulling this "glass" (which is now a thirty-four foot long tube of course) any higher, must result in a pure vacuum at the top of the tube, and I do mean pure. There wouldn't be a single atom of matter in this space, with the exception of matter vaporizing off of the inside of the tube or off of the surface of the water (which is physically unavoidable. A tungsten container would vaporize very very little). I'm not really sure what the reaction of these materials would be to the presence of a pure vacuum (which would of course make the materials frigidly cold except for heat transmitting through the water and container from the outside). Once this vacuum is attained, sealing it off so that it can be used is easy. Simply slide a door across the tube right above the water level (at thirty-four feet high), and the vacuum is now contained (to the extent that the container doesn't leak). It can now be transported, or otherwise used in whatever fashion is desired. If the materials making up a desired object that uses a vacuum can be submerged under water without suffering damage, these materials could be pushed up through the tube of water into the vacuum before the door is closed off. Now the materials can be constructed into their desired form in a vacuum, which of course means the object itself can contain a small piece of this vacuum if a vacuum is needed in the object, or in some cases, certain materials are so sensitive to matter of any kind that a vacuum is the only place nonvolatile enough to work with them. For example, oxygen has horrible corrosive effects on a wide variety of materials. |